Treating is a process used to produce cleaner gasoline which helps protect the environment and human health, gasoline molecules contain impurities like sulfur that can be removed when the molecule is heated and come in contact with a specific catalyst a chemical reaction occurs that strips the sulfur away. these sulfur compounds can be used in fertilizer and pharmaceuticals.

HOW THE PROCESS WORKS
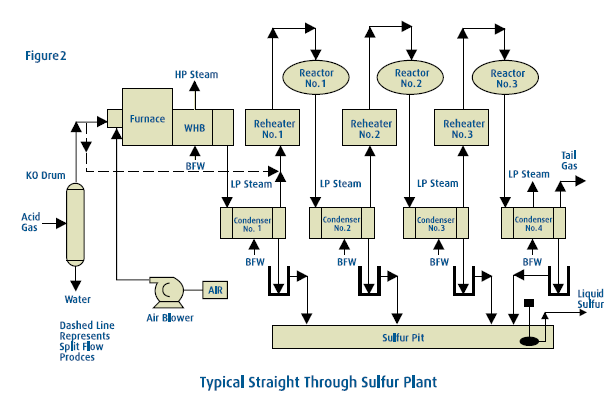
Feed gas for a Claus Sulfur Recovery Unit usually originates in an acid gas sweetening plant. The stream, containing varying amounts of H2S and CO2, is saturated with water and frequently has small amounts of hydrocarbons and other impurities in addition to the principal components. In a typical unit, H2S-bearing gas enters at about 8 psi and 120°F. Combustion air is compressed to an equivalent pressure by centrifugal blowers. Both inlet streams then flow to a burner which fires into a reaction furnace. The free-flame Modified Claus reaction can convert approximately 50% to 70% of the sulfur gases to sulfur vapor. The hot gases, up to 2,500°F, are then cooled by generating steam in a waste heat boiler. The gases are further cooled by producing low-pressure steam in a separate heat exchanger, commonly referred to as a sulfur condenser. This cools the hot gases to approximately 325°F, condensing most of the sulfur which has formed up to this point. The resultant liquid sulfur is removed in a separator section of the condenser and flows by gravity to a sulfur storage tank. Here it is kept mol-ten, at approximately 280°F, by steam coils. Sulfur accumulated in this reservoir is pumped to trucks or rail cars for shipment.
TECHNOLOGIES
We offer the following for sulfur recovery and tail gas cleanup:
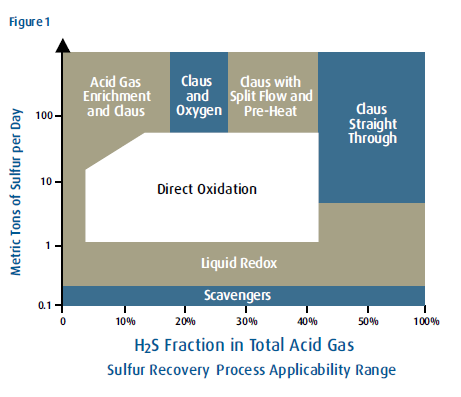
Straight-Through Claus/Split-Flow Claus/Direct Oxidation/Acid Gas Enrichment/Oxygen Enrichment /Cold Bed Adsorption/Shell Claus off-gas Treating (SCOT)
Catalytic Reaction Completes The Process
Any further conversion of the sulfur gases must be done by catalytic reaction. The gas is reheated by one of several means and is then introduced to the catalyst bed. The catalytic Claus reaction releases more energy and converts more than half of the remaining sulfur gases to sulfur vapor. This vapor is condensed by generating low-pressure steam and is removed from the gas stream. The remaining gases are reheated and enter the next catalytic bed. This cycle of reheating, catalytic conversion and sulfur condensation is repeated in two to four catalytic steps. A typical SRU has one free-flame reaction and three catalytic reaction stages. Each reaction step converts a smaller fraction of the remaining sulfur gases to sulfur vapor, but the combined effect of the entire unit is to reduce the hydrogen sulfide content to an acceptable level.
High Yields Plus Energy
Claus sulfur plants can normally achieve high sulfur recovery efficiencies. For lean acid gas streams, the recovery typically ranges from 93% for two-stage units (two catalytic reactor beds) up to 96% for three-stage units. For richer acid gas streams, the recovery typically ranges from 95% for two-stage units up to 97% for three-stage units. Since the Claus reaction is an equilibrium reaction, complete H2S and SO2 conversion is not practical in a conventional Claus plant. The concentration of contaminants in the acid gas can also limit recovery. For facilities where higher sulfur recovery levels are required, the Claus plant is usually equipped with a tail gas cleanup unit to either extend the Claus reaction or capture the unconverted sulfur compounds and recycle them to the Claus plant. All Claus SRU’s produce more heat energy as steam than they consume. This is particularly true for those plants equipped with waste heat boilers on the incinerator. The steam produced can be used for driving blowers or pumps, reboiler heat in the gas treating or sour water stripping (SWS) plants, heat tracing, or any of a number of other plant energy requirements.