
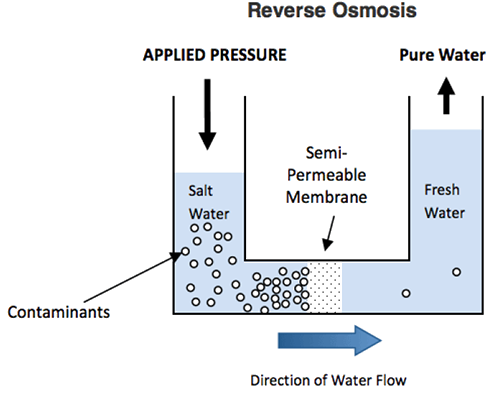
When pressure is applied to the concentrate solution, the water molecules are forced to flow through the semi-permeable membrane (The contaminants are left behind since the semi-permeable membrane is only permeable to water molecules and not permeable to ions and other contaminants).
How does reverse osmosis work?
In practice, reverse osmosis is applied as a flow filtration process.
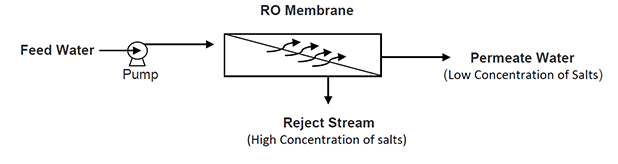
A high-pressure pump is used to increase the pressure on the origin of the RO and force the water to flow across the semi-permeable membrane., leaving most part (approximately 95%-99%) of the dissolved salts behind the stream.
Within the membrane system, the feed water will be split into a low-saline product, called permeate, and a high saline solution called concentrate, brine or reject stream.
Applications of RO system | l Municipal drinking water |
l Food and beverage industry | |
l Agricultural irrigation | |
l Industrial ultrapure water | |
l Industrial process water | |
l Wastewater reuse | |
l Power industry (boiler feed water, cooling towers) | |
l Municipal/industrial water reuse | |
l Households |